Motor oil deteriorates during its life in the engine due to oxidation. This results in sludge, varnish and resins that become deposited on engine surfaces. Deposits in the piston ring belt area cause ring sticking, loss of compression and increased oil consumption. Deposits can also block oil lines and passages which prevent the oil from reaching parts that need to be lubricated. The results are increased wear, heat build-up and eventual engine failure.
Engine oil is also contaminated with fuel soot because of incomplete combustion of the fuel as well as carbon which is introduced into the engine by emission control systems – diesel engine oil in particular. Oil viscosity increases with soot loading. High oil viscosity leads to cold-start problems and risk of oil starvation. When the soot concentration reaches a level that can no longer be suspended in the oil, the soot precipitates out of the oil to form sludge and deposits. High concentrations of soot also lead to increased wear.
To control all these contaminants, engine oils are formulated with detergents and dispersants in the performance additive package. Antiwear agents, rust inhibitors, and antioxidants are also incorporated in the performance package. In addition, multigrade oils contain viscosity index improvers. The viscosity index improver additive cannot be included in the performance package and is mixed into the oil separately. Pour point depressants and foam inhibitors are also included in the oil formulation, normally blended into the oil as separate components.
The performance package is dominated by the detergent and dispersant components. Considering the large amounts of contaminants the oil must handle (soot particles in particular) these two additives normally make up between 60% and 80% of the performance package. The terms ‘detergent” and ‘dispersant’ are often used interchangeably because the two additives work in synergy to keep engines clean, but the way they function is completely different.
Detergents are oil soluble organo-metallic compounds, mostly derived from the organic soaps or salts of calcium, magnesium or sodium, with calcium being the most commonly used. They have polar heads which allow them to cling to metal surfaces. Detergents serve two principal functions. Firstly, they remove deposits from metal surfaces inside the engine. Deposits and metal surfaces are both polar and deposits are drawn to the metal surfaces and stick to them. The detergent, with its stronger charge, displaces deposits from the metal surface as shown in Fig 1. Secondly, detergents are highly alkaline and neutralize acids formed in the oil by chemically reacting with them to form harmless neutralized chemicals.
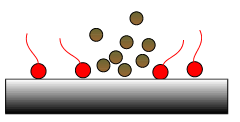
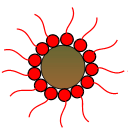 Fig 2: Dispersants hold deposits in suspension.
Fig 2: Dispersants hold deposits in suspension.
Fig 1: Detergents remove deposits
from metal surfaces.
Due to their metallic nature detergents are prone to produce residues and ash when burned in the engine.
Dispersants are polar additives that dissolve sludge and soot to prevent them from agglomerating, settling out and forming deposits. Dispersant molecules consist of an electrically charged polar head and a long, oil soluble tail. The polar heads attract and ‘embrace’ potential deposit forming materials and acids which are taken into
solution in the oil by the tails as illustrated in Fig 2. Dispersants do not contain any metallic elements. If they are burned in the engine, they do not leave any residue or ash.
Due to their alkaline nature, detergents and dispersants contribute to the alkalinity reserve or Total Base Number (TBN) of engine oil. Of these two additives, detergents add the most to TBN. Dispersants are more rapidly depleted than detergents because of the way they react with contaminants and acids in the oil.
Detergents, on the other hand, have the ability to retain their alkalinity reserve over longer periods of time, thus providing better TBN retention. We mentioned earlier that detergents produce ash when burned in the engine (due to their metallic nature) and therefore they contribute to the SAPS (Sulphated Ash, Phosphorus and Sulphur) level of engine oil.
Nowadays the main driving force for the development of new engine oils is concern over the environmental impact of engine emissions. Current generation lubricants must provide optimum exhaust gas emission control system durability. To protect these systems, engine oils must contain lower SAPS levels since SAPS can poison emission control after-treatment devices. The reduction in oil SAPS limits has resulted in a shift from traditional engine oil technologies to alternative low ash additive chemistries and there is now increased focus on detergents and dispersants derived from polybutenes.


