Doctors have perfected the art of conducting physical exams. They know what questions to ask and how to examine the body for clues that signify health, injury or disease. Maintenance staff, lubrication technicians and even equipment operators should be competent to perform ‘physicals’ as well. Like the doctor, they need to be aware of subtle changes or symptoms that might be an early sign of machine malfunction or accelerated wear.
One of the obvious problems with conducting such inspections is that for most machines, the critical operating components, including the lubricant itself, are shielded from view by panels, casings, guards and housings. It is like asking your doctor to give you a physical while wearing body armour. Nevertheless, the machine and the lubricant can telegraph hints and signals in various ways and the selection of machine inspections should be tailored to the machine design and operating environment. This is done by selecting which inspections are needed and how frequently they should be carried out.
Following is a list of problem-revealing tests and inspections related to lubrication. They require limited technical proficiency and most of the tests involve no special tools or instruments.
Colour Change:
Monitor changes in lubricant colour with sight glasses and oil samples. Oils that have been subjected to high temperatures will exhibit marked changes (darkening) in colour and opacity. Many types of contaminants will alter colour as well. Additionally, a wrong lubricant can often be recognized by a change in darkness or colour.
Air Release Capability:
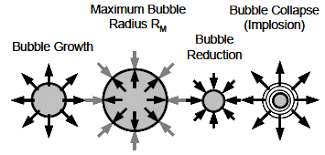
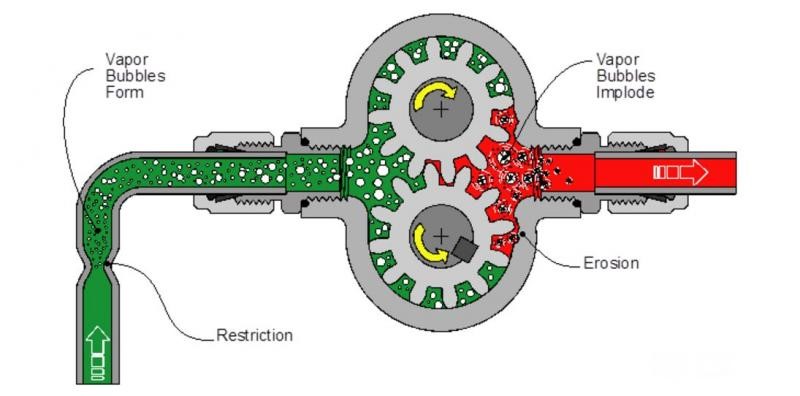
Most healthy lubricants will release entrained air readily. Distressed and contaminated lubricants may fail to release air from the oil and may also form sustained surface foam. Aeration and foaming (see OilChat 49) are not always oil related and may point to air entering the system or mechanical problems. Sight glasses and inspection hatches may provide the first sign of a problem.
Water Shedding Ability:
Water and oil usually do not mix. However, if they become conjoined in an emulsion, the problem is often associated with a change in the oil properties or contamination. Observing oil/water separation after violently mixing quantities of each in a sample bottle or laboratory glassware is an easy check for this property.
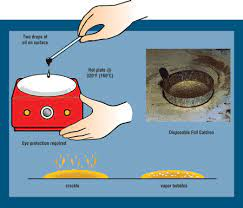
Blotter Spot Test:
This inspection involves placing a drop of oil on blotter paper and then observe how the oil spot ‘develops’. Healthy oil does not produce any structures and will spread out evenly on the paper, leaving a uniform gradient of oil colour behind. When the oil is badly contaminated, serrated patterns may form or the contaminants can clump together and do not migrate with the oil front. The Blotter Spot Test was discussed in detail in OilChat 72.
Sediment and Water:
It is often said that what is bad for the oil or material that has been removed from machine surfaces is also heavier than the oil. We know that substances heavier than the oil will settle out when mixed with the oil. That is why there is great wisdom in inspecting for water and sediment using drain port sampling.
Noise:
Few machines are quiet during operation. Even completely healthy machines have something to say. You may have heard the saying “a singing gear is a happy gear”. This is often true, but not always. Machine sounds change for specific reasons. Try to locate the point of generation. Some maintenance technicians play doctor by using crude stethoscopes in the form of a garden hose, a screwdriver or a steel rod held to their ear, with the other end touching potential noise-generating areas.
Temperature:
Doctors use thermometers but maintenance technicians employ a variety of tools including online temperature probes/thermocouples, thermal imaging cameras or handheld heat guns. A few common causes of temperature changes are wrong lubricants, degraded lubricants, contaminated lubricants, abnormal friction/wear – and the list goes on.
Pressure Change:
In the same way oil temperature can change in response to an assortment of problems, oil pressure can increase or decrease as well. Anything that can change viscosity or form surface deposits can change system pressure. For similar reasons it is no surprise that doctors pay special attention to blood pressure during an exam.
Filter Life:
There usually is a good reason when filters plug prematurely and it is worthy to investigate what is plugging the filter. Areas of particular concern are soft contaminants (sludge, organic material, dead additive residue, etc.), dust and wear debris. The filter is the final resting place for a variety of machine and lubricant operational waste products as discussed in OilChat 76.
You should have noted the many interesting human health analogies above, but this is just a start. There are far too many field tests and inspections to discuss in a publication like this. For those who make a living caring for the health of lubricants and machinery, the value of being capable to perform machine physicals is immense. Backed by laboratory oil analysis and other predictive maintenance technologies, the modern-day lubrication engineer and condition-monitoring professional is indeed fortunate to have such a ‘clear’ view of the internal state and operational health of his machinery. If you have any queries about Machine Health Checks, please email us at info@bcl.co.za. Our lubricant experts will be happy to answer any questions you may have.







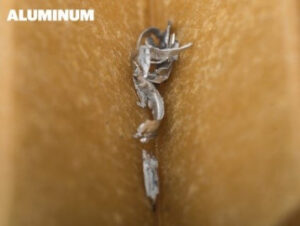 Many modern engines use lightweight aluminium components and pinpointing the origin of shiny aluminium particles in the oil filter can be difficult. One area that experiences regular wear is the piston skirt. Abnormal wear of the piston skirts is often due to contamination that has entered the combustion chamber or the use of the wrong engine oil. Checking the air intake and filter system is a good idea if you believe contamination has entered the engine.
Many modern engines use lightweight aluminium components and pinpointing the origin of shiny aluminium particles in the oil filter can be difficult. One area that experiences regular wear is the piston skirt. Abnormal wear of the piston skirts is often due to contamination that has entered the combustion chamber or the use of the wrong engine oil. Checking the air intake and filter system is a good idea if you believe contamination has entered the engine. Other small, shiny particles that can be confused with aluminium are tin, lead and copper. These materials are often used in the Babbitt alloy layer of the main and piston rod small end bearings. Insufficient lubrication of these bearings can lead to premature wear of the Babbitt layer and eventually to the failure of the bearing. If remnants of tin, lead or copper are found, it would be wise to ensure that the engine is being properly lubricated with the correct oil.
Other small, shiny particles that can be confused with aluminium are tin, lead and copper. These materials are often used in the Babbitt alloy layer of the main and piston rod small end bearings. Insufficient lubrication of these bearings can lead to premature wear of the Babbitt layer and eventually to the failure of the bearing. If remnants of tin, lead or copper are found, it would be wise to ensure that the engine is being properly lubricated with the correct oil. In many cases, you will find some amount of carbon, which is difficult to identify, but the use of a magnifying glass can help. When rubbing carbon between your fingertips it will feel slightly gritty but it will break apart easily. Carbon accumulation is typically a result of blow-by getting past the piston rings. If your engine suffers from excessive oil consumption and low cylinder compression readings, you most probably have worn or damaged piston rings.
In many cases, you will find some amount of carbon, which is difficult to identify, but the use of a magnifying glass can help. When rubbing carbon between your fingertips it will feel slightly gritty but it will break apart easily. Carbon accumulation is typically a result of blow-by getting past the piston rings. If your engine suffers from excessive oil consumption and low cylinder compression readings, you most probably have worn or damaged piston rings.
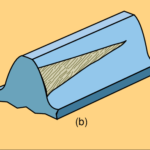
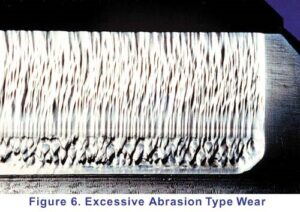 Abrasion can range from mild to severe. Very mild abrasion may cause polishing of gear teeth. Moderate abrasion consists of fine scratches with little removal of material from the contact surfaces. Severe abrasion manifests itself as deep grooves on gear teeth as shown on the right. Abrasive wear is brought about by particle contamination of the oil or rough gear surfaces as discussed below:
Abrasion can range from mild to severe. Very mild abrasion may cause polishing of gear teeth. Moderate abrasion consists of fine scratches with little removal of material from the contact surfaces. Severe abrasion manifests itself as deep grooves on gear teeth as shown on the right. Abrasive wear is brought about by particle contamination of the oil or rough gear surfaces as discussed below: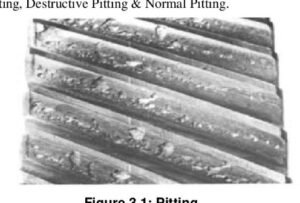 The formation of pits or holes on gear teeth is called pitting and is caused by overload conditions on the gear tooth surface. Severe pitting is also referred to as spalling. A pit forms when small cracks (due to overloading) grow long enough to separate a piece of material at the surface. These irregularities cause additional friction and result in a lot of heat being generated, which in turn reduces the viscosity of the oil. A possible solution would be to use an EP oil with higher viscosity.
The formation of pits or holes on gear teeth is called pitting and is caused by overload conditions on the gear tooth surface. Severe pitting is also referred to as spalling. A pit forms when small cracks (due to overloading) grow long enough to separate a piece of material at the surface. These irregularities cause additional friction and result in a lot of heat being generated, which in turn reduces the viscosity of the oil. A possible solution would be to use an EP oil with higher viscosity. Scuffing or scoring can be described as grooves over a wide area of gear teeth. Scuffing leads to the much more serious wear mechanism referred to as galling as seen on the left. It occurs when a lack of lubrication causes metal-to-metal contact of gear teeth, resulting in elevated temperatures. This can result in localized welding in contact areas. Metal is then ripped from one surface and transferred to the other. This type of wear is also referred to as adhesive wear.
Scuffing or scoring can be described as grooves over a wide area of gear teeth. Scuffing leads to the much more serious wear mechanism referred to as galling as seen on the left. It occurs when a lack of lubrication causes metal-to-metal contact of gear teeth, resulting in elevated temperatures. This can result in localized welding in contact areas. Metal is then ripped from one surface and transferred to the other. This type of wear is also referred to as adhesive wear.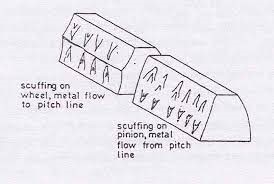 On driven gears metal ‘flows’ towards the middle of the gear teeth, along the length of gear teeth, as shown above and on the left gear tooth in the adjacent diagram. On driving gears metal propagates away from the centre line of the gear as depicted on the far right. Possible solutions would be to use an EP oil with a higher viscosity and reducing the temperature of the oil.
On driven gears metal ‘flows’ towards the middle of the gear teeth, along the length of gear teeth, as shown above and on the left gear tooth in the adjacent diagram. On driving gears metal propagates away from the centre line of the gear as depicted on the far right. Possible solutions would be to use an EP oil with a higher viscosity and reducing the temperature of the oil.


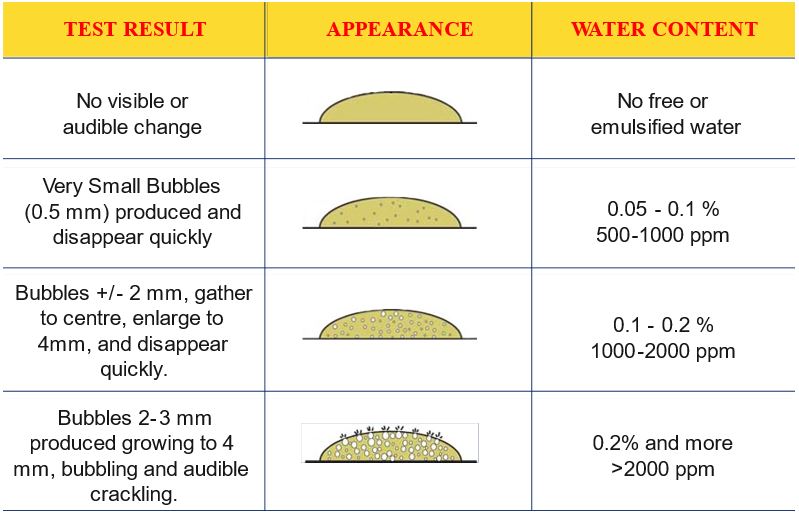
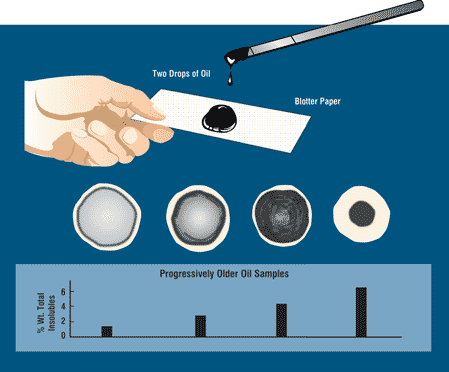


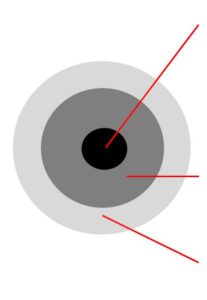


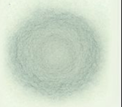
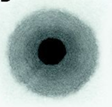




 y the contaminant band should spread with the oil to leave a fairly even discoloration. When the oil is badly contaminated, the contaminants clump together and do not migrate with the oil front. This indicates that the dispersancy additive of the oil is depleted.
y the contaminant band should spread with the oil to leave a fairly even discoloration. When the oil is badly contaminated, the contaminants clump together and do not migrate with the oil front. This indicates that the dispersancy additive of the oil is depleted.